Understanding Absolute Ethanol: Purity, Properties, and Applications

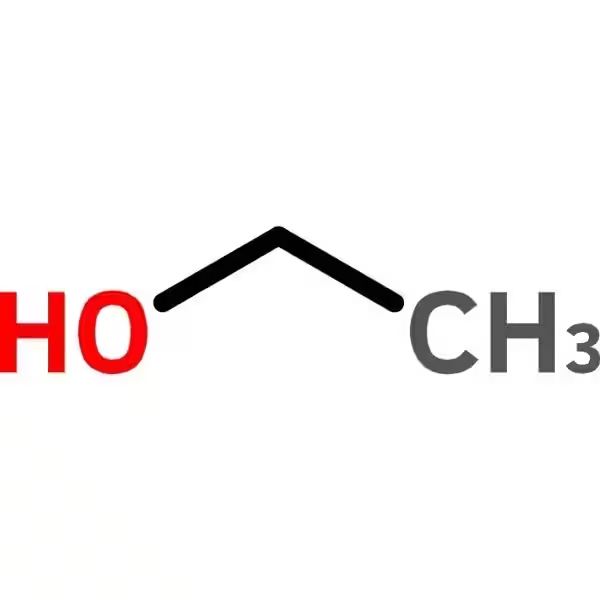
Absolute ethanol, also known as absolute alcohol or 100% ethanol, is a pure form of ethyl alcohol (C₂H₅OH) lacking significant amounts of water or other impurities. This high purity sets it apart from other grades of ethanol, making it crucial for specific applications. This article delves into the properties, uses, and safety considerations associated with absolute ethanol.
Chemical and Physical Properties of Absolute Ethanol
Absolute ethanol is a colorless, volatile, and flammable liquid with a characteristic odor. Its purity is its defining characteristic, differentiating it from other ethanol grades which may contain water, methanol, or other impurities. These impurities can affect the properties and applications of the alcohol.
The absence of significant water content gives absolute ethanol unique characteristics. Its high purity allows for precise control in its various applications, minimizing the interference of other substances. This high purity is especially important in scientific, pharmaceutical, and certain industrial processes where even small amounts of impurities can significantly affect the outcome.
Production and Purification of Absolute Ethanol
The journey from fermented sugars to absolute ethanol involves several steps. Initially, yeast ferments sugars, producing a dilute ethanol solution. Subsequent distillation is used to raise the concentration of ethanol. However, achieving absolute ethanol (100% purity) requires further purification techniques.
Traditional distillation alone cannot achieve absolute ethanol because ethanol and water form an azeotrope—a mixture with a constant boiling point. To break this azeotrope and remove the remaining water, methods like azeotropic distillation (using a third component to alter the boiling point) or the use of desiccants (drying agents) are employed. The choice of method depends on the desired level of purity and economic factors. The resultant product is absolute ethanol, which is highly pure, devoid of significant water content, and often used in highly sensitive applications.
Applications of Absolute Ethanol: A Diverse Range
The high purity of absolute ethanol makes it suitable for numerous applications across various sectors. Its versatility stems from its excellent solvent properties, and its lack of impurities makes it suitable for use in applications requiring high purity.
Absolute Ethanol as a Solvent
Absolute ethanol's superior solvency makes it a valuable solvent in numerous industries. In the pharmaceutical industry, it serves as a solvent for medicine preparation and extraction. The cosmetics industry uses it extensively as a solvent and carrier for various ingredients. It's also employed in the production of paints and varnishes, where its ability to dissolve both polar and nonpolar substances is crucial. Its purity makes it ideal for applications requiring high-level precision, as the absence of contaminants ensures consistent results.
Absolute Ethanol in the Fuel Industry
Absolute ethanol finds use as a biofuel, a renewable alternative to fossil fuels. It can be used as an additive to motor gasoline (gasohol), or, in some countries, as a pure fuel source for vehicles. The use of absolute ethanol as a fuel shows the versatility of the chemical, highlighting its value as a sustainable alternative to traditional energy sources. It’s important to note that the use of ethanol as a fuel does not come without considerations regarding environmental impact and potential for engine damage compared to gasoline.
Absolute Ethanol in Scientific Research
In scientific research and laboratories, absolute ethanol is essential for various techniques. It's a common solvent in chromatography and spectroscopy, where its high purity minimizes interference with analytical results. Its use in DNA and RNA purification is also notable due to its ability to precipitate nucleic acids and separate them from other cellular components. It is vital for studies requiring high purity and accuracy.
Absolute Ethanol in Medical Applications
While ethanol in beverages is well known, pure absolute ethanol has important medical uses. It is a sterilizing agent, used in some disinfectant solutions and hand sanitizers for its ability to eliminate microorganisms. However, it's crucial to note this must be at appropriate concentrations; pure absolute ethanol is not recommended for direct use as a disinfectant, and any use should follow established protocols.
Absolute Ethanol in other applications
Beyond these major uses, absolute ethanol has niche applications in various industries. Its use as a reagent in chemical synthesis, as a preservative, and in various industrial processes highlights its versatility and importance in diverse sectors. The high purity eliminates interference from other substances making it reliable and predictable in various chemical reactions.
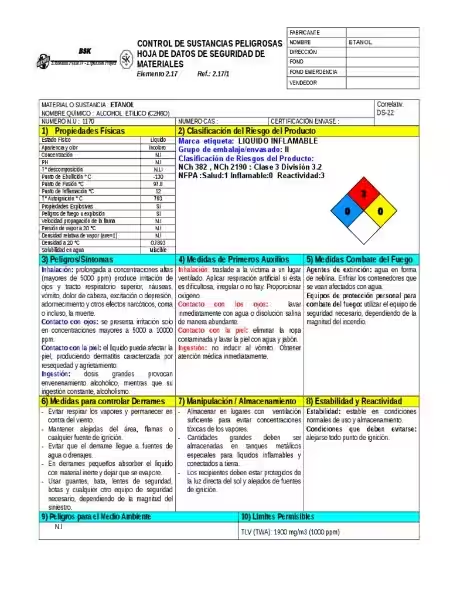
Safety and Handling of Absolute Ethanol
Absolute ethanol presents certain hazards, including its high flammability. Even a small spark can ignite its vapors, demanding careful handling and storage in well-ventilated areas. Direct contact can cause skin irritation, and ingestion is highly toxic.
Safety precautions when handling absolute ethanol include:
- Using appropriate personal protective equipment (PPE), such as gloves and eye protection.
- Working in a well-ventilated area to prevent the buildup of flammable vapors.
- Storing it in a cool, dry place, away from ignition sources.
- Proper labeling, consistent with safety regulations.
- Familiarization with and adherence to relevant safety data sheets (SDS).
In conclusion, absolute ethanol is a versatile substance with a wide range of applications. Its high purity is essential for its various uses, making it a crucial component in various scientific, industrial, medical, and even recreational contexts. However, its inherent flammability and toxicity demand adherence to strict safety protocols during handling and storage to prevent potential hazards. Understanding both its benefits and risks are crucial for safe and effective use.
Frequently Asked Questions about Absolute Ethanol
What is absolute ethanol?
Absolute ethanol refers to ethanol (ethyl alcohol) with a purity of 99.5% or higher. The remaining percentage is typically water. It's considered anhydrous, meaning it's essentially free from water. This high purity distinguishes it from other grades of ethanol, such as denatured alcohol or rectified spirits.
What are the chemical and physical properties of absolute ethanol?
Absolute ethanol is a colorless, volatile, and flammable liquid with a characteristic odor. Key properties include:
- High solubility: It readily dissolves in water and many organic solvents.
- Low boiling point: It boils at 78.37 °C, facilitating its separation from water through distillation.
- Lower density than water: This difference in density aids in separation techniques.
- High flammability: Requires careful handling and storage due to its significant fire hazard.
- Toxicity: While used in beverages, pure absolute ethanol is highly toxic if ingested in large quantities.
How is absolute ethanol produced?
Absolute ethanol production typically begins with the fermentation of sugars by yeast. This process yields a mixture of ethanol and water. To achieve the high purity of absolute ethanol, further purification steps are necessary. These often involve sophisticated distillation techniques, possibly including azeotropic distillation, which helps to remove the remaining water, along with other methods such as adsorption or membrane separation to remove any trace contaminants.
What are the main applications of absolute ethanol?
Absolute ethanol's high purity makes it suitable for a wide range of applications:
- Solvent: In pharmaceuticals, cosmetics, and paints, its ability to dissolve various substances is crucial.
- Pharmaceuticals: Used as a solvent and preservative in many medicines. Its high purity is essential for pharmaceutical applications.
- Fuel: Employed as a biofuel, alone or blended with gasoline.
- Scientific research: Frequently used as a solvent and reagent in laboratory settings. Its purity is critical for accurate results.
- Disinfectant (in specific concentrations): Its antimicrobial properties are utilized in disinfectants and antiseptics, however, the concentration is critical for efficacy.
Is absolute ethanol safe to handle?
No, absolute ethanol is not safe to handle without proper precautions. Its flammability poses a significant fire risk, and ingestion is highly toxic. Always handle absolute ethanol in a well-ventilated area and use appropriate personal protective equipment (PPE), such as gloves and eye protection. Follow all safety guidelines provided by the manufacturer and relevant safety data sheets (SDS).
What are the differences between absolute ethanol and other grades of ethanol?
Absolute ethanol differs from other grades primarily in its purity. Denatured alcohol contains additives to make it unsuitable for consumption, while rectified spirits have a lower ethanol concentration. The purity level dictates the suitability of the ethanol for specific applications; absolute ethanol's high purity is essential where contamination would be detrimental.
How should I store absolute ethanol?
Store absolute ethanol in a tightly sealed, approved container in a cool, dry, well-ventilated area, away from ignition sources and incompatible materials. Keep it away from direct sunlight and heat. Always follow the manufacturer’s storage recommendations. Proper labeling is crucial for safety.
What happens if I accidentally ingest absolute ethanol?
Accidental ingestion of absolute ethanol can be extremely dangerous and potentially fatal. Immediately seek medical attention. Do not induce vomiting unless instructed by medical professionals. Provide emergency personnel with details about the amount ingested.