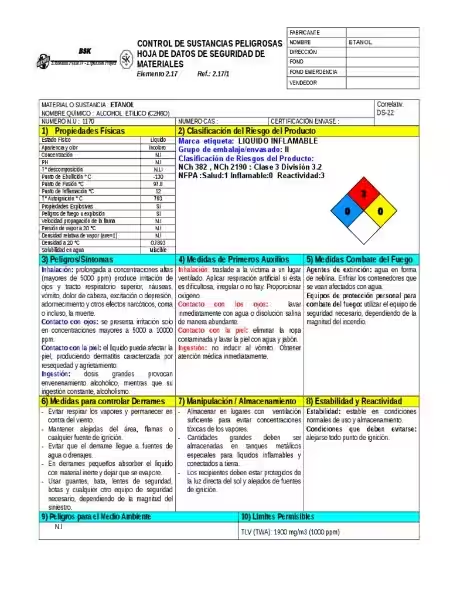
Understanding the Ethanol Hazard Diamond: A Comprehensive Guide

Understanding the hazards associated with ethanol is crucial for anyone handling this common chemical. While often perceived as relatively benign due to its presence in alcoholic beverages, ethanol possesses significant fire and health risks that warrant careful consideration. This article will delve into the specifics of ethanol's hazards, focusing on the information conveyed through the ethanol hazard diamond, a visual representation of its inherent dangers. We will explore its flammability, reactivity, appropriate safety measures, and emergency response procedures.
Flammability and Fire Hazards
Ethanol is highly flammable, a key aspect highlighted prominently on the ethanol hazard diamond. Its low flash point of 55°F (13°C) means it ignites easily at relatively low temperatures. This makes it a significant fire hazard, especially in enclosed spaces or when dealing with large quantities.
The vapors of ethanol are heavier than air, meaning they tend to sink and accumulate in low-lying areas. This increases the risk of explosions, particularly in poorly ventilated environments. Furthermore, flashback along vapor trails is a possibility, extending the potential reach of a fire. Therefore, proper ventilation is paramount, and ignition sources must be strictly controlled.
Fire Suppression Techniques
Extinguishing ethanol fires requires specialized methods. Water spray alone may be ineffective, as it can spread the burning liquid. For small fires, dry chemical extinguishers, CO2 extinguishers, alcohol-resistant foam, or carefully applied water spray can be effective.
Larger fires demand a more cautious approach. Water spray or fog, or alcohol-resistant foam, are generally preferred, but it's vital to avoid direct streams onto the burning liquid in large containers. Fighting fires involving large containers should be done from a maximum safe distance or using unmanned firefighting devices. Even after the fire is extinguished, containers must be cooled extensively to prevent reignition.
Reactivity and Chemical Incompatibilities
The ethanol hazard diamond doesn't always fully represent the chemical reactivity of ethanol. While the pure substance is relatively stable, it reacts violently with certain chemicals. This reactivity is a critical safety concern.
Mixing ethanol with strong oxidizing agents such as concentrated hydrogen peroxide is particularly dangerous, potentially leading to the formation of powerful explosives. Reactions with other substances including acetyl chloride, acetyl bromide, concentrated sulfuric acid, and hypochlorous acid can also produce unstable and explosive compounds. Even contact with a platinum-black catalyst can trigger ignition. Therefore, careful selection of storage containers and avoidance of incompatible materials is crucial. Cellulose-based absorbents, often used for spill cleanup, should be avoided due to their potential to react.
Safe Handling and Storage
To minimize the risk of reactions, ethanol should be stored in appropriate containers, away from incompatible chemicals. Storage areas should be well-ventilated to prevent the buildup of flammable vapors. Clearly labeled containers and a comprehensive safety data sheet (SDS) readily available are essential. Regular inspections should be conducted to identify potential hazards and ensure proper handling procedures are followed. Moreover, employees should receive thorough training on the safe handling and storage of ethanol.
Health Hazards and Personal Protective Equipment (PPE)
While liquid ethanol isn't considered acutely toxic, its vapors can irritate the eyes, nose, and throat. Prolonged or high-concentration exposure can lead to more severe health effects. The ethanol hazard diamond should be a reminder of this.
Skin contact should also be avoided. While not typically causing severe immediate damage, prolonged exposure can lead to skin dryness and irritation. Wet clothing contaminated with ethanol is highly flammable and must be removed immediately. The use of appropriate PPE, including safety glasses or goggles, gloves, and protective clothing, is essential when handling ethanol.
Choosing the Right PPE
The selection of PPE depends on the specific task and the potential exposure level. For many applications, standard chemical-resistant gloves and safety glasses are sufficient. However, for high-risk operations or large spills, more robust protective clothing, such as DuPont Tychem® suits, may be necessary. It's important to note that even specialized suits offer limited protection near heat or flames. Regular inspection and replacement of damaged PPE is essential.
Emergency Response Procedures
Spills of ethanol must be addressed immediately. The required isolation distance depends on the spill size and the presence of fire. A minimum of 50 meters (164 feet) is recommended for smaller spills. The distance increases to 300 meters (984 feet) for larger spills and a staggering 800 meters (2625 feet) for fires involving tanks.
Evacuation of personnel may be necessary, depending on the severity of the incident. Spill response should focus on eliminating ignition sources, preventing entry into confined spaces, and using appropriate absorbent materials for cleanup. Remember, never use cellulose-based materials. Appropriate absorbents designed for flammable liquids should be used.
Dealing with Ethanol Spills
For smaller spills, the use of inert absorbents, followed by careful disposal according to local regulations, is recommended. Larger spills require a more coordinated response, potentially involving emergency services. Proper training and a well-defined emergency response plan are critical for handling ethanol spills effectively and minimizing potential risks. Regular drills can ensure that personnel are prepared to react quickly and efficiently in case of an emergency.
The ethanol hazard diamond serves as a visual reminder of the potential dangers associated with this commonly used chemical. Understanding its flammability, reactivity, and health hazards, along with appropriate handling procedures and emergency response protocols, is crucial for ensuring workplace safety. By taking the necessary precautions and adhering to best practices, the risks associated with ethanol can be effectively mitigated. Remember, even though ethanol might seem relatively harmless in some contexts, its inherent properties demand respect and caution.
Ethanol Hazard Diamond FAQ
Here are some frequently asked questions about the hazards associated with ethanol, as represented on a hazard diamond:
What are the primary hazards associated with ethanol?
Ethanol's primary hazard is its high flammability. It has a low flash point (55°F), meaning it ignites easily. Its vapors are heavier than air, increasing the risk of explosions in confined spaces, and flashback along vapor trails is possible. While the liquid itself isn't considered acutely toxic, its vapors can irritate the eyes, nose, and throat. Furthermore, ethanol reacts violently with certain chemicals, creating potentially explosive mixtures (e.g., with concentrated hydrogen peroxide).
What does the hazard diamond for ethanol indicate?
The specific values on the hazard diamond would vary depending on the concentration and the specific regulatory system used, but generally, it would highlight the significant flammability hazard (high numerical rating). It may also indicate a minor health hazard due to the irritant nature of the vapors. Reactivity would depend on the presence of incompatible materials. No specific hazard would be expected for special hazards.
What are the fire safety precautions for ethanol?
Ethanol fires require specialized extinguishing techniques. Water spray might be ineffective for larger fires; dry chemical, CO2, alcohol-resistant foam, or water spray/fog are better options, depending on the fire size. For large containers or tank fires, fight the fire from a maximum safe distance or use unmanned devices. Always cool containers extensively after the fire is extinguished, even if it seems out. Prevent ignition sources near ethanol storage or handling areas.
What personal protective equipment (PPE) is necessary when handling ethanol?
Appropriate eye protection is essential, as is protective clothing to prevent skin contact. Wet clothing should be removed immediately due to flammability. Specialized suits, like DuPont Tychem® (depending on the specific fabric), offer enhanced protection against breakthrough, but should never be used near heat or open flames.
What is the emergency response procedure for an ethanol spill?
Immediate isolation of the spill area is crucial, with minimum isolation distances varying based on spill size (50 meters for small spills, 300 meters for large spills, and 800 meters for fires involving tanks). Evacuation may be necessary. Eliminate all ignition sources. Use appropriate absorbent materials for cleanup, avoiding cellulose-based materials due to their reactivity with ethanol. For large spills, the advice of emergency services and hazardous materials teams is strongly recommended.
What first aid measures should be taken in case of ethanol exposure?
For eye contact, immediately flush with water for 20-30 minutes and seek medical attention. For skin contact, flood the affected area with water, remove contaminated clothing, and thoroughly wash the skin. For inhalation, move the person to fresh air and seek medical attention if symptoms (eye, nose, throat irritation) develop. If ingested, immediately seek medical attention; do not induce vomiting.
What are the key physical properties of ethanol relevant to its hazards?
Ethanol's key properties include its low flash point (55°F), its vapor density (1.59, heavier than air), its boiling point (173.3°F), its lower explosive limit (LEL) of 3.3%, and its upper explosive limit (UEL) of 19%. These properties contribute significantly to its flammability and potential for explosion. Its autoignition temperature is 689°F. Its miscibility with water also affects cleanup and handling strategies. The IDLH (Immediately Dangerous to Life or Health) concentration is quite high (3300 ppm).
What are the chemical reactivity hazards of ethanol?
Ethanol reacts violently with certain chemicals, including acetyl chloride, acetyl bromide, concentrated sulfuric acid, and strong hydrogen peroxide. Mixtures with concentrated hydrogen peroxide can form powerful explosives. Reactions with hypochlorous acid and chlorine produce ethyl hypochlorite, which is unstable and potentially explosive. Contact with a platinum-black catalyst can also cause ignition. Incompatible materials should be avoided during storage and handling.